Overview
Dilute bacteria or phage to 10-x dilution.
Materials
- 96-well microtiter plate
- Burner & Striker
- Gloves
- 300µL pipette and sterile tips
- Sterile 0.86% Saline solution
- Sterile Reservoir
- Bacteria or Phage needed to dilute
Protocol
- Clean your bench with 70% ethanol and put on your gloves
- Place all needed materials on your bench
- Turn on the gas and light the burner
- Pour some sterile 0.86% saline into the reservoir
- Using a Multichannel pipette, pipette 270µL saline from reservoir into all or appropriate wells of microtiterplate (it is fine to reuse tips for this step) [Note: for students of Biology 481/563, this step and previous step will be done for you]
For diluting a single column:
- Label Column 1 with what you will be diluting
- Pipette 30µL of bacteria into well A1, discard tip (please see Figure 1 below for a zoomed-in schematic about how to move your bacteria from the source to this destination well)
- Using a new tip, gently mix well A1 3-4 times, and transfer 30µL from well A1 to well B1
- Using the same tip (if there is no residual liquid in it), gently mix well B1 3-4 times, and transfer 30µL from well B1 to well C1
- Continue diluting using the same tip until you have reached the target dilution (note that if the tip collects bubbles or touches a potentially contaminated surface-- dispose of it, load a new sterile tip, and continue the dilution series; please see Figure 2 below for a zoomed-in schematic about the steps involved in a dilution series).
You are now ready to use your diluted bacteria or phage for the next step of your protocol
Notes and Caveats
- Transferring from the initial culture into well A1 is counted as a 10-1 dilution. Thus, we would say that well B1 contains a 10-2 dilution, well C1 contains a 10-3 dilution, and so on. It is often helpful to write the numbers "-1", "-2", "-3", and so on, on the far left of the lid of the microtiter plate to keep track of which dilution well you aspirate from (e.g., for plating).
- Important: When handling a microtiter plate always keep the plate parallel with the floor (turning the plate so that it forms an angle with the floor can cause the wells to spill and can lead to cross-well contamination).
Figure 1
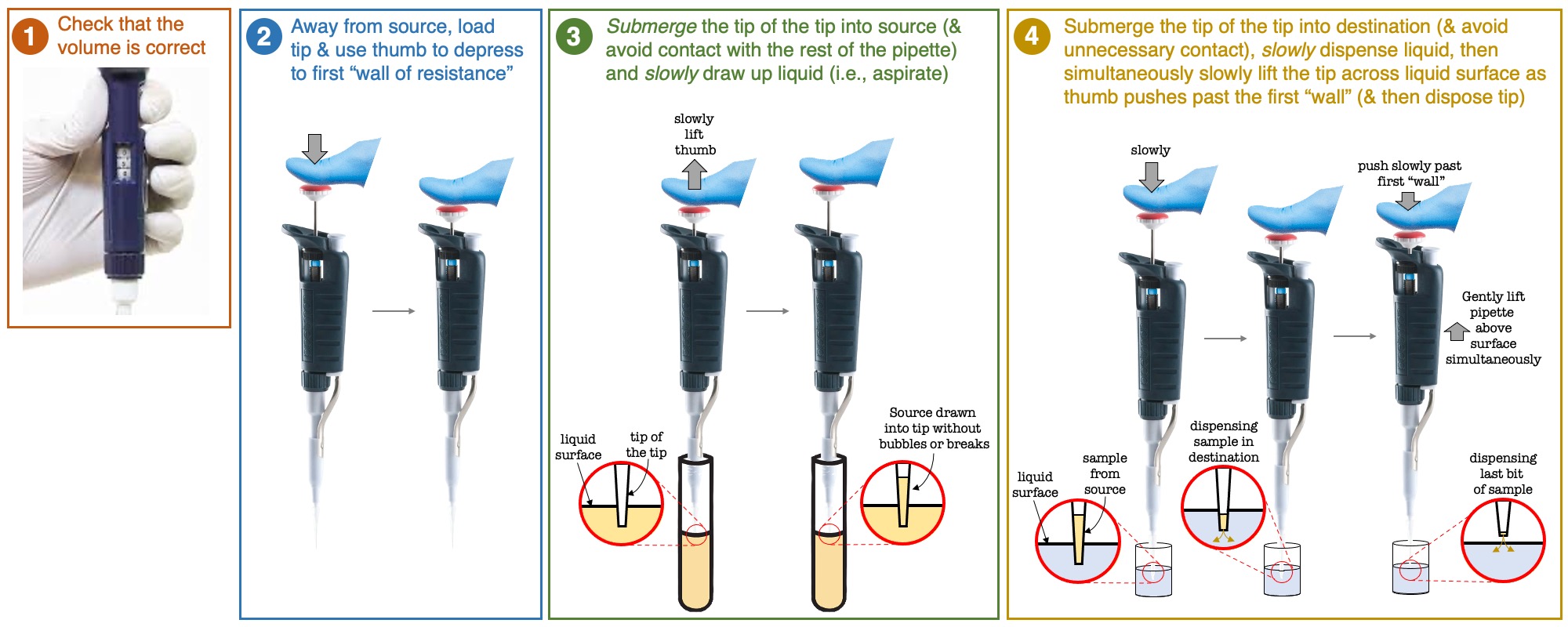
Figure 1: The steps to pipetting from a source (e.g., containing bacteria) to a destination (e.g., a well with saline)
Figure 2
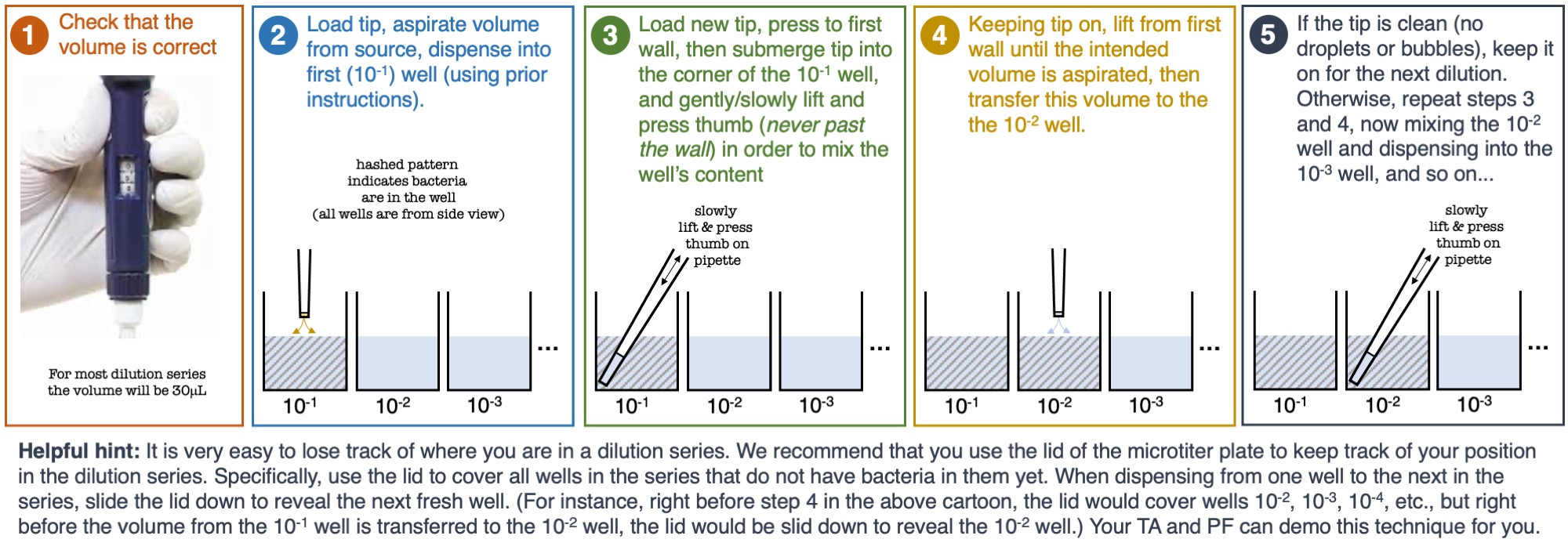
Figure 2: The steps to performing a dilution series over a set of wells filled with saline